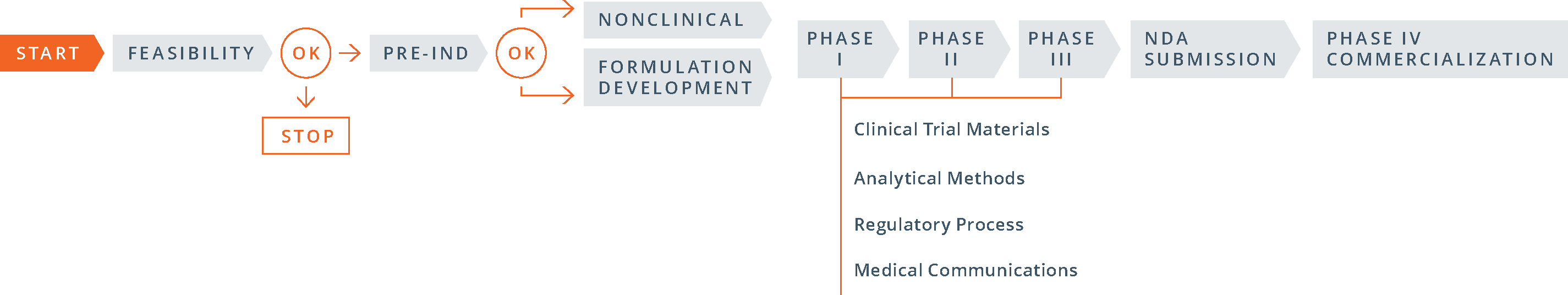
USA - Determining Whether to Submit an ANDA or a 505(b)(2) Application - Guidance for Industry - RIS.WORLD
Citizen Petitions and Petitions for Stay of Action Subject to Section 505(q) of the Federal Food, Drug, and Cosmetic Act Guidanc
Postmarketing Studies and Clinical Trials—Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act
Citizen Petitions and Petitions for Stay of Action Subject to Section 505(q) of the Federal Food, Drug, and Cosmetic Act